Why choose Lithium-Titanate (LTO)?
What is Lithium-Titanate (LTO) technology?
An LTO battery is named after its key component, lithium titanium oxide (LTO) powder. The material is also referred to as lithium titanate with the chemical formula Li4Ti5O12. Unlike most lithium-ion battery materials it is used as the anode active material. LTO anodes are used in electric vehicle batteries and mobile medical devices due to the high level of safety and recharge capabilities.
Advantages of LTO Batteries
LTO is used instead of carbon materials like graphite as the anode active material due to a variety of reasons including:
High Intercalation Potential
Higher lithium intercalation potential of LTO compared to graphite so Li ions are more likely to contribute to the charging and discharging.
Great Rate Capability
175mAh g-1 theoretical capacity reached without compromising cycle life and very high rates (800C) reached.
Zero Strain Material
Very little change in volume of the crystal when lithium ions are intercalated and when they are not.
Good Cycle Stability
Zero strain nature increases the amount of time the battery can be charged and discharged, known as their cycle life.
High Li+ Diffusion
Lithium ions can also diffuse through LTO more efficiently than graphite. This means the rates of charge and discharge are much quicker.
Great Safety
with both conventional and low-temperature electrolytes
But why is it safer than conventional lithium-ion batteries?
LTO batteries contain lithium-titanium-oxide as anode material instead of graphite. The use of titanium improves the cycle life of the battery and has a better temperature performance, compared to other lithium-ion counterparts
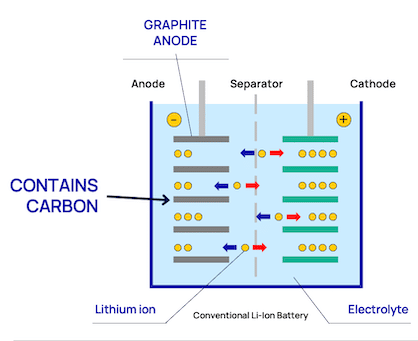
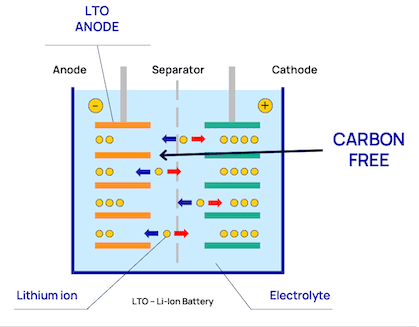
By using lithium-titanium-oxide in the anode, we achieve a number of positive characteristics, which apart from making it possible to build compact and durable systems, also have implications for battery safety. These are:
No dendrite build-up and no risk for ruptured isolator
Almost zero swelling during charge-discharge (no volume changes)
Rapid charging and low-temperature performance
Self-healing mechanism that prevents short circuit in case of penetration
Let's go through them all.
No dendrite build-up on the anode
Dendrite build up is one of the major problems for lithium-ion batteries and is also the causes for undetectable internal short-circuits. This is a sinister and complex battery degradation problem, because it grows slowly over time within the battery cell and can't be detected.
Unlike NMC, LTO do not have problem with dendrite build up. This is because of its anode material (lithium-titanium-oxide), that doesn't contain carbon. Conventional lithium-ion batteries usually have graphite anodes containing carbon and are more prone to dendrite build-up over time.
Dendrites are a branch-shaped metallic lithium, that are formed when the anode is exposed to overvoltage (when potential drops to 0 V vs Li/Li+). With conventional lithium-ion batteries that uses carbon in the anode, lithium-ion insertion (when lithium ions get absorbed into a solid) happens when the potential is close to 0.1 V vs Li/Li+. So even just a slight overvoltage (0 V vs Li/Li+ compared to 0,1 V vs Li/Li+) increases the likelihood of dendrite deposits. This exposure can happen when charging rapidly or charging in cold temperatures for example.
The likelihood of this happening in an LTO anode is very low. In LTO anodes, lithium-ion insertion happens when the electric potential is at 1.5 V vs Li/Li+, (compared to 0 V vs Li/Li+). This provides a comforting margin.
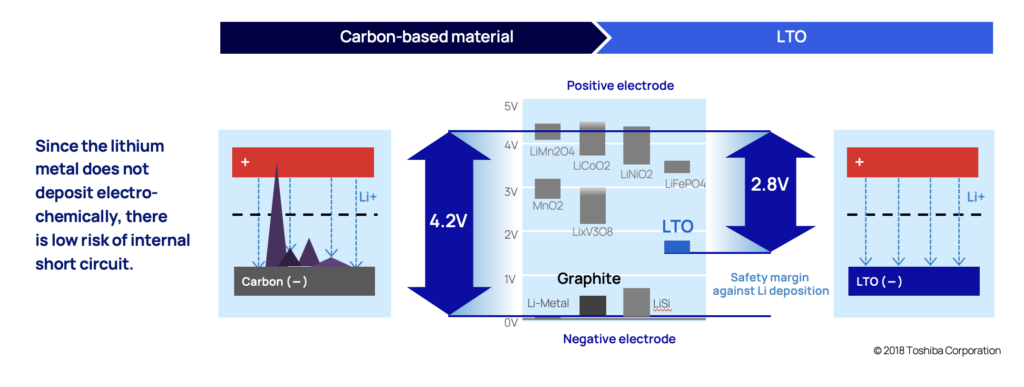
The slow continuous dendrite build-up also causes battery cells to degrade quicker than desired which is also the primary reason most battery chemistries won't last as many cycles as LTO. As dendrites grow, they eventually reach the cathode by piercing through the thin material separating the anode and the cathode (called separator), and the battery short circuits.
Minimal volume changes during charge and discharge
Almost all lithium-ion batteries expand and decompress as charge goes in vs out from the battery - i.e., absorbing and releasing lithium ions. All batteries experience some level of physical volume changes when they are charged or discharged (i.e., absorbing or releasing lithium ions). This physical volume change, depending on how dramatic it is, can damage the battery's material and will over time, lower its capacity and shorten its lifespan and potential cycle life.
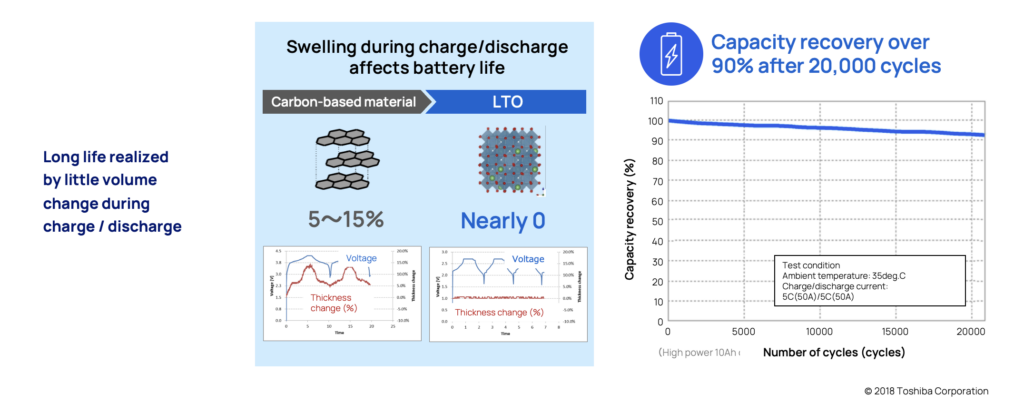
Many common chemistries can experience volume change of around 5-15 percent. LTO which are structured in what's called a spinel shape, make them very stable and experience virtually zero change in volume. This is also one of the primary reasons the battery has such a long life span.
To verify this, tests have been performed on LTO batteries under extreme circumstances. After more than 20,000 cycles in 35°, the battery still held over 90% of its capacity.
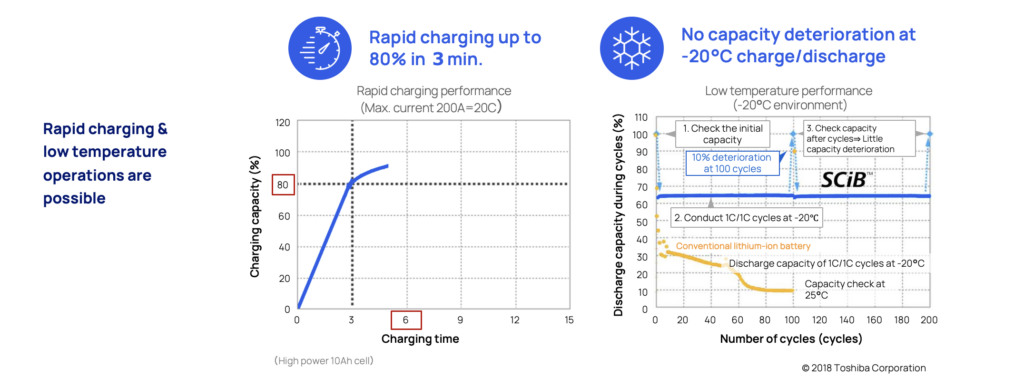
Rapid charging and low-temperature performance
The LTO battery is perfect when there's a need for safe and super fast charging. In three minutes (3) it can be charged up to 80 percent without causing dendrite build up that eventually will degrade the battery and increase the risk for internal short circuits.
The unique anode material is also resilient to extreme temperatures. In as low as -20° there is still no sign of dendrite build up or degradation. This makes LTO optimal for extreme maritime applications such as naval vessels, or commuter traffic in varying climates such as the Nordics and in hot and humid climate zones.
Self-healing mechanism that prevents short circuit in case of penetration
Short circuits in batteries often leads to catastrophic events, with a rapid thermal runaway event as the most likely outcome. One of the key advantages of LTO is that is has a self-protection mechanism against the dramatic effects of short-circuits.
External threats to battery systems are something to be cautious of. In maritime settings, humidity, and saltwater poses as a general risk for batteries, and for naval vessels a stray bullet or force towards the battery cells can lead to fires and explosions.
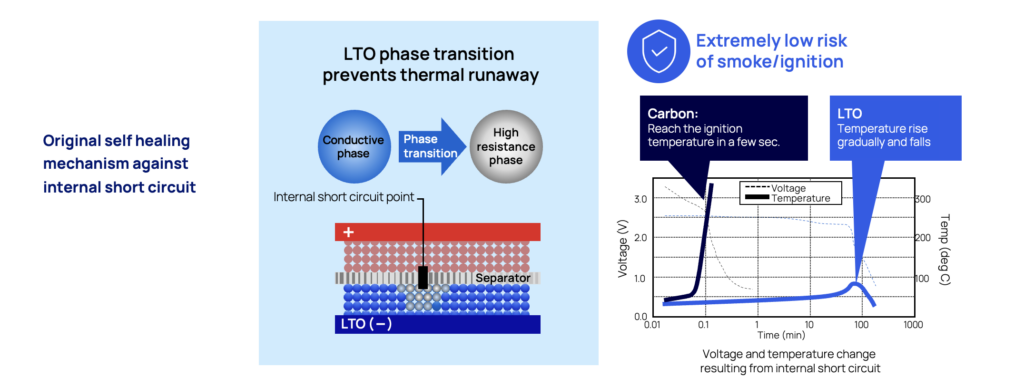
If this would happen - seeing that an object gets in between the cathode and anode by accident, the lithium ions are released from the anode (the LTO part) adjacent to the point of short circuit and will immediately turn the LTO anode into an insulator. By reacting like this, the initial thermal runaway will be controlled and stopped by preventing the flow of short circuit currents.
LTO vs LiFePO₄ - Technology Comparison
Both Lithium-Titanate (LTO) and LiFePO₄ are safe lithium battery technologies, but they are optimized for very different priorities.
| Feature | LTO (Lithium-Titanate) | LiFePO₄ |
|---|---|---|
| Charging speed | Ultra-fast charging and discharging 10-15 minutes | 2-4 hours |
| Cycle life | 30,000+ cycles | 2,000-5,000 cycles |
| Operating temperature | -40 °C to +60 °C | -20 °C to +50 °C |
| Safety | Extremely high | High |
| Energy density | Lower (50-80 Wh/kg) | Higher (90-160 Wh/kg) |
| Upfront cost | Higher | Lower |
| Best use case | High-cycle, extreme, critical systems | Weight- and cost-sensitive systems |